Abstract
This bioprinting roadmap features salient advances in selected applications of the technique and highlights the status of current developments and challenges, as well as envisioned advances in science and technology, to address the challenges to the young and evolving technique. The topics covered in this roadmap encompass the broad spectrum of bioprinting; from cell expansion and novel bioink development to cell/stem cell printing, from organoid-based tissue organization to bioprinting of human-scale tissue structures, and from building cell/tissue/organ-on-a-chip to biomanufacturing of multicellular engineered living systems. The emerging application of printing-in-space and an overview of bioprinting technologies are also included in this roadmap. Due to the rapid pace of methodological advancements in bioprinting techniques and wide-ranging applications, the direction in which the field should advance is not immediately clear. This bioprinting roadmap addresses this unmet need by providing a comprehensive summary and recommendations useful to experienced researchers and newcomers to the field.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
Corrections were made to this article on 13 May 2020. Figure 6 and figure 7 were replaced better quality images.
1. Introduction
Wei Sun1, 2
1Drexel University, Philadelphia, PA 19104, United States of America
2Tsinghua University, Beijing, People's Republic of China
For hundreds of years, cells have been understood to be Nature's building blocks that make us what we are. Can we use the same building blocks to fabricate biological models that will be able to help cells to work? Can the fabricated models and therapeutic products help us better study biology, target cancers, and develop new drugs?
As a core technology in biofabrication, bioprinting utilizes cells, proteins and biomaterials as building blocks for 3D-printed biological models, biological systems and therapeutic products. This emerging technique has rapidly evolved according to the use of functional building blocks, for example, from printing biomaterials for tissue scaffolds and implants, to printing cells or organoids for 3D biological models, and to printing micro-organ-chips for microphysiological platforms and engineered living systems, such as cellular machining and biorobots (figure 1). Applications of biomedical 3D printing have also advanced from early surgical planning models, inert implants, and cell-seeded scaffolds to in vitro bioprinted models. Bioprinting makes available the study of in vitro regenerative and physiological function, disease and pathogenesis development (including cancer), and drug screening with intended in vitro cell or tissue models. These broad applications have also stimulated the development of novel bioinks, translational tissue engineering, personalized cancer treatments, and drug discoveries.
Figure 1. Advances in bioprinting.
Download figure:
Standard image High-resolution imageNotwithstanding the advancements in bioprinting techniques there are a number of challenges which must be overcome. The main challenges include, but are not limited to, (1) bioinks: the need for a new generation of novel bioinks with multifunctional properties to better transport, protect, and grow cells during and after printing; (2) printing process: better printing processes and printers to deliver cells with high survivability and high precision; (3) crosslinking: efficient and effective crosslinking techniques and crosslinkers to maintain bioink structural integrity and stability after printing; (4) long-term cell culture: integration with microfluidic devices to provide a long-term and a simulated physiological environment in which to culture printed models.
This bioprinting roadmap reports key advances in the selected areas, highlighting the status of current developments and challenges, as well as the envisioned advances in science and technology, to address these challenges in the field of bioprinting. Topics covered in this roadmap represent the broad spectrum of bioprinting; from cell expansion and novel bioink development with novel rheological, material, and/or mechanical properties to cell/stem cell printing, from organoid-based tissue organization to bioprinting of human-scale tissue structures, and from building cells/tissues/organs-on-a-chip to biomanufacturing of multicellular engineered living systems with functional vasculature. These systems are reported as already in use for rapid drug screening, such as for cancer therapies. Another common need that is discussed is bioreactors with gas and waste product concentration sensing and control, as well as electrical, chemical, or mechanical stimulation capabilities. The emerging application of printing-in-space (microgravity) and an overview of biofabrication 3D printing technologies are also included in this roadmap. Several authors cover the need for higher-resolution 3D printing, defined media to insure final structure and functional potency, and biofabrication of devices with higher cell densities in existing and yet to come biofabrication technologies. There is also debate over extracellular matrix (ECM), growth factor, and substrate properties versus cells being relied on to retain stemness or unfold their fate and develop sufficient vascularization and innervation without preformed or newly formed structures for vasculature and/or critical functions. Due to the rapid pace of methodological advancements in bioprinting techniques and wide-ranging applications, the direction in which the field should advance is still evolving. The bioprinting roadmap aims to address this unmet need by providing a comprehensive summary and recommendations useful to experienced researchers and newcomers to the field.
2. From cell expansion to 3D cell printing
Binil Starly and Edward P Fitts
Department of Industrial and Systems Engineering, Comparative Medicine Institute, North Carolina State University, Raleigh, NC 27695
2.1. Status
A key component of any biofabrication process is the living cells, often required in the 10 million–20 million cells per ml quantities, with the need to reach cell densities greater than 200 million cells for any potent post-biofabricated tissue or organoid structure. Yet, the majority of laboratory-based processes for generating these cells are dependent on flat culture plate solutions to generate the millions of cells needed to bioprint tissue. Biofabrication systems are generally considered a mid-stream operational step in a process that begins from the tissue biopsy until the last step of delivery of the product to the patient [1]. Cell expansion is a critical upstream process step for cell and tissue manufacturing. Bioreactor-based systems to expand cells for both autologous and allogeneic-based therapies are being considered to replace the time-consuming step of producing enough cells. Further improvement in bioreactor-based cell-expansion systems is required to lower barriers to the adoption of bioprinting in regenerative medicine and tissue engineering product markets.
Many of the bioreactor systems currently in use today are derived from those that have been in use for the biopharmaceutical-based production of vaccines, viral vectors, and monoclonal antibodies [2]. However, the design of these bioreactors has not considered the fact that for biofabrication, the cells are the living product. When cells are the product of the cell-expansion system, critical care must be taken during the expansion process to preserve the cellular properties required for needed functionality in the biofabricated product. While the science of cell expansion and design of tissue systems have progressed over the last two decades, the manufacturing science of the process to 'scale-up' and 'scale-out' to cost-effectively manufacture these products has not received significant attention and remains a key barrier to market readiness [3]. Progress has been made towards cell-expansion reactors that address the low wall shear requirement of mesenchymal stem cell (MSC) expansion, albeit towards 'scaling-up' the number of cells [4, 5]. More work is to be carried out towards automating workflows during a scale-out operation when patient-derived cells from multiple patients are expanded simultaneously for economically viable production.
2.2. Current and future challenges
As biofabrication progresses towards translational research, a holistic view of how the cellular and tissue product transforms from one step to the next is critical for addressing the quality of biofabricated products.
- Tissue products that result from biofabrication are dependent on the growth of adherent cells. Most adherent cells used as the key ingredient in cell bioprinters are derived from passaging through standard or cell-stack-based flat culture plates [6]. These are inherently 2D forms of culture which will require substantial amounts of floor space to grow the necessary therapeutic amounts. This translates to expensive good manufacturing practice (GMP) facilities leading to very expensive cell and regenerative medicine therapy procedures.
- Current commercially available bioreactors fall short of being able to adequately monitor the cellular conditions for expanding cell populations in situ and in real-time. A key technical hurdle is the 'black-box' nature of available bioreactor systems for automated cell expansion. Operators of bioreactor systems during a three week to four week expansion phase have limited information rich in-process data on the status of the expanding cells until they are brought out of the chamber for offline sample analysis. Current methods resort to frequent media sampling which are prone to error and can get prohibitively expensive in autologous cell-expansion culture.
- Variability in characteristics of the incoming cell population can necessitate changes to the expansion phase of the culture [7, 8]. A standard operating procedure may not always work optimally for expanding patient-specific cells. Experienced technicians are required to adjust culture conditions within regulated limits for optimal output. This can add to cost, time and potential loss of quality.
- A general lack of understanding exists on the effect of the various processing conditions on the end quality profile of the cells prior to loading within bioprinters. Several phase I clinical trials are being conducted with the use of therapeutic cells/tissues. With many of them having the potential to move to phase II trials and beyond, it is critical to understand and standardize upstream and downstream processing steps that affect the potency of tissue products.
2.3. Advances in science and technology to meet challenges
Several new technology platforms can enable solutions to the challenges above. From a basic manufacturing science perspective, new studies must be conducted to understand changes in the cellular machinery as the expansion process takes place and how each population doubling affects cellular potency. Further, tissue-based therapies that involve the use of patient-specific cells, how the variation in cellular profile after the expansion process affects the biofabricated product must also be understood. Several related technology platforms can be utilized to help meet these challenges.
- New culture platforms must be integrated within cell-expansion bioreactors to improve the cellular yield per unit volume. This is possible through improving the culture surface area utilizing 3D surfaces, new bioreactor designs specific to autologous and allogeneic therapy, culture media that enhances cellular growth rates, etc. Improving cellular yield will drive higher efficiency through which cells are produced for the biofabrication market and improve scale-out operations.
- Cell-expansion bioreactors are complex hydrodynamic environments with a complex multiphysics environment, involve chemical kinetics, flow-induced reaction, and a living cellular material expanding under the right conditions. New hardware systems to determine cell number, viable cell mass, cellular status (for example, undifferentiated or differentiated) in real-time and in-process without negatively disturbing the growth rate of the cells can significantly advance cell-expansion-based operations. Real-time and label-free sensors are required to quantify key performance indicators (KPI) to allow quality-by-design approaches to be implemented for both cell-expansion reactors and associated biofabricated systems [9, 10].
- New computational models must be developed that integrate real-time sensing, and control strategies that adapt to expanding cell populations based on cellular characteristics will be critical for improving the robustness of the manufacturing process. Gathering experimental data from cell expansion and biofabricated systems can be very expensive and distributed across various laboratories, start-up companies, and large companies. Academia across the world can lead the way to interconnecting hardware systems through a global cyber-infrastructure that allows data-sharing and management to lower the barriers for those involved in developing new hardware and software-based regenerative medicine products.
2.4. Concluding remarks
The science behind the scale-up and scale-out of cell manufacturing and tissue biofabrication is critical to the adoption of these processes in therapeutic and non-therapeutic applications. Mass production of tissue/organoids for allogenic therapy or non-therapeutic applications will require cell-expansion systems that produce massive quantities of cellular products, with processes moving away from labor-intensive processes to production scale methods. For autologous therapy of personalized biofabrication products, new challenges of quality control and scale-out production strategies must be devised for economic viability. New cyber-physical technology combined with innovative artificial-intelligence-based learning algorithms built into biological machines and advances in materials development can perhaps address many of the technical challenges posed by engineered tissue/organoid manufacturing technology.
Acknowledgments
The author would like to acknowledge the support of US NSF Grant# US NSF #1562139 and funds from the NC State Comparative Medicine Institute.
3. Bioinks for 3D bioprinting
Andrew C Daly1, Jürgen Groll2 and Jason A Burdick1
1Department of Bioengineering, University of Pennsylvania, Philadelphia, PA, United States of America
2Chair of Functional Materials in Medicine and Dentistry, University of Würzburg, Würzburg, Germany
3.1. Status
Biofabrication technologies hold great promise for developing in vitro models or implantable constructs that mimic the complexity of native tissues and organs [11]. Biofabrication encompasses a broad range of manufacturing processes, including bioprinting, where cells, biomaterials, and biologically active factors are printed into 3D constructs [11]. Bioprinting inherently requires a bioink, which can be defined as 'a formulation of cells suitable for processing by an automated biofabrication technology that may also contain biologically active components and biomaterials' [12]. The term 'bioink' was originally introduced along with the term 'biopaper' with the advent of organ printing in 2003 [13]. Initially, the term referred to a purely cellular component (i.e. cellular spheroids) patterned onto a hydrogel biopaper. With advances in extrusion and lithography bioprinting, the term was expanded to include cell-containing hydrogels that are processed with these technologies to fabricate 3D structures.
The design and application of bioinks has expanded greatly in the last decade, with numerous materials—primarily natural and synthetic hydrogels—being applied or developed to meet the stringent demands of bioprinting [14]. Whether processed through extrusion (material extruded from a nozzle, sometimes termed bioplotting) or lithography (spatial control of material crosslinking with light) bioprinting, materials must transition from fluid to solid states at the appropriate time. The rheological properties of the bioink are crucial to the success of the biofabrication process, and the bioink and processing steps must support the encapsulation and maintenance of viable cells [14]. Although significant progress has been made in engineering bioinks for bioprinting, further advances in the field will facilitate the encapsulation of cells in microenvironments that better mimic the complexity of native tissues and organs, particularly through the incorporation of new signals and by processing at increased resolutions. In this section we will first discuss current and future challenges for the field. Next, we will discuss how recent advances in biomaterial science and bioprinting technology have helped address these challenges. Finally, we will highlight some important unaddressed challenges for the field.
3.2. Current and future challenges
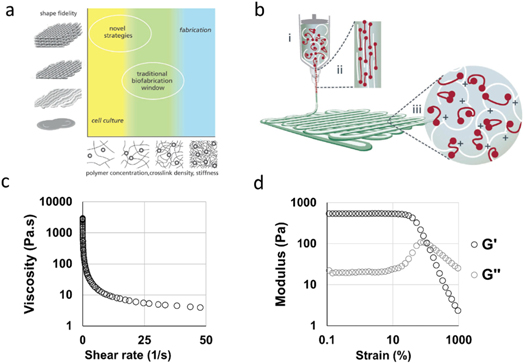
The major challenges in the design of bioinks for bioprinting include: (i) designing materials that can be processed with current or developing biofabrication techniques at desired resolutions, (ii) maintaining the viability of cells during and after processing, and (iii) providing the appropriate cellular environment to guide desired cell behaviour. Balancing printability with cell viability and function has been challenging, as important cellular processes, such as proliferation, differentiation, and ECM deposition, can be impeded when cells are embedded in dense polymer networks, while dense networks often support the best shape-fidelity and long-term stability after printing (figure 2(a)) [14].
Figure 2. Bioinks for extrusion bioprinting. (a) An outline of the traditional biofabrication window for extrusion bioprinting, where denser hydrogels are required for optimal extrusion characteristics (fabrication window) and softer hydrogels are required to support biological processes such as cell proliferation, differentiation, and ECM production (cell-culture window). The traditional biofabrication paradigm has focused on developing hydrogel bioinks with properties in the region between these two windows, whereas novel bioink strategies are being developed that exist in both the cell-culture and fabrication windows [14]. (b) A schematic of shear-thinning behaviour during extrusion of a bioink containing reversible crosslinks. (i) In the syringe the polymer chains form a temporary crosslinked network. (ii) Upon dispensing through a needle the temporary network is broken through shear and the viscosity is decreased to facilitate flow. (iii) Upon deposition, shear stresses are removed and the reversible crosslinks reform to enable filament localisation [14]. (c) Representative rheological properties of a shear-thinning bioink showing decreasing viscosity with increased shear rate, and (d) shear-yielding (G' and G'' crossover at approximately 85% strain) with increased strain (0 to 1000 %, 1 Hz) (unpublished data, Burdick lab).
Download figure:
Standard image High-resolution imageExtrusion bioprinting is the most widely used technique and requires bioinks that can flow through a nozzle and then remain localised upon deposition, traditionally through extrusion in a low-viscosity state and then rapid stabilization upon deposition with additional crosslinking. For example, gelatin-methacryloyl (GelMA) bioinks have been widely used in the field, as they can be stabilised upon extrusion by cooling the printing platform to induce physical gelation or by photocrosslinking in the presence of initiators and light [11, 14]. In contrast to purely liquid-to-solid transitions, another approach is the use of shear-thinning hydrogels that possess reversible crosslinks that disassemble under shear and self heal when shear forces are removed to enable filament localisation [14] (figures 2(b)–(d)). Shear-thinning bioinks can be prepared using high molecular weight polymers and/or polymer concentrations, or through the use of engineered reversible crosslinks [14].
A range of lithography-based biofabrication technologies, such as stereolithography (SLA), digital light processing (DLP), and 2-photon polymerization (2PP) have been developed for photo-patterning cell-laden hydrogels into complex 3D geometries [11]. These approaches involve layer-by-layer spatial patterning of light to photocrosslink specific regions of a bioink (acting as a resin) composed of a low-viscosity photocrosslinkable hydrogel precursor [15, 16]. Although lithography bioprinting often offers greater resolution than extrusion bioprinting, challenges include long processing times when fabricating thick constructs for tissue engineering applications, maintaining cells in a suspended state during fabrication for uniform cell resolution, and incorporating more than one material/cell within the printed construct.
3.3. Advances in science and technology to meet challenges
There are two major approaches that are taken in the advancement of bioinks in bioprinting to address the stated challenges, namely, (i) the development of new printing processes that support the processing of previously developed materials and bioinks, or (ii) the design of new bioinks that can be processed using current bioprinting processes. Both must consider the final cell environment and print resolution and whether these are sufficient for the intended application.
Towards the first approach, a number of smart bioprinting strategies have been developed that permit the controlled extrusion of low-viscosity hydrogels. For example, low-viscosity methacrylated hyaluronic acid (HA) and GelMA bioinks can be extruded as stable filaments using a transparent nozzle that enables photocrosslinking during extrusion, prior to deposition [17]. Another approach for formulating such materials into printable bioinks is the addition of a sacrificial polymer to stabilise the bioink during extrusion. For example, alginate has been widely utilized for these purposes as it can be rapidly crosslinked during extrusion in the presence of calcium and is easily washed out post-printing (figure 3(a)) [18].
Figure 3. Advances in bioinks for extrusion and lithography bioprinting. (a) A schematic demonstrating a widely used coaxial crosslinking technique, where a low-viscosity alginate/GelMA bioink is included in the core region and calcium chloride solution is included in the shell region. When the two solutions come into contact at the needle tip the alginate is immediately ionically crosslinked to stabilise the filament. Next, the GelMA bioink is covalently crosslinked with UV light, and the alginate component is then washed out using a mild chelating agent [18]. (b) A schematic outlining extrusion of a bioink into a granular support material composed of Carbopol microparticles (0.2 μm) that locally fluidize around the printing nozzle during writing to trap the printed filament within 3D space, and an example of a printed thin-shell octopus model printed using the technique [19]. (c) High-resolution DLP photolithography of solid pyramid, cone, and flower structures with feature sizes of 50–500 μm through improved bioink formulation. The bioink is composed of methacrylated poly (vinyl alcohol) (PVA-MA), GelMA, and a RU (tris-bipyridyl- ruthenium (II) hexahydrate)-SPS (sodium persulfate) photo-initiator [15]. (d) Microfluidic enabled DLP lithography bioprinting of diverse multimaterial cellular hydrogels with high-resolution features [16].
Download figure:
Standard image High-resolution imageAnother promising approach involves printing bioinks into granular support hydrogels that fluidize around the printing needle during extrusion and subsequently solidify to trap the printed structure in 3D space [19]. For example, low-viscosity bioinks such as poly (vinyl alcohol) (PVA), which are challenging to process using traditional layer-by-layer extrusion, can be extruded into complex 3D geometries using granular support baths that provide stabilization of the printed structure and can later be removed (figure 3(b)) [19]. Similarly, shear-thinning hydrogels can be deposited into the 3D space of another hydrogel, including with multiple bioinks across a wide range of patterns [20]. These approaches allow increased complexity within 3D environments.
Advances are also being made in lithography bioprinting, such as the transition to newer photoinitiators (e.g. transitional metal-based photoinitiators) to reduce irradiation intensities and times to speed up printing [15]. When combined with photo-absorbers to limit off-site curing, such high-resolution features (<25 μm) can be obtained [15] (figure 3(c)). In addition to speeding up crosslinking, strategies that accelerate replenishment of the bioink between successive polymerization steps will also help reduce fabrication times. Recently, multibioink lithography approaches have also been developed by integrating a microfluidic inlet with a DLP bioprinter that allows switching of the bioresin during printing to produce heterogeneous constructs [16] (figure 3(d)).
3.4. Concluding remarks
Advances in bioink development and processing, ideally addressed simultaneously in the future, will enable the further development of bioprinting techniques to realize their true potential towards the fabrication of in vitro models, or for the development of implantable constructs for tissue repair. Many questions are yet unanswered, such as the needed resolution and material complexity to achieve a functional product, which will certainly vary for different target tissues and research questions. For sure, bioinks must not only meet the demands of the bioprinting process (e.g. extrusion, lithography), but they need to provide suitable environments for cells. Future advances will be needed in our understanding of how the bioink controls cell behaviour to actively orchestrate cells towards correct functional tissue maturation, together with a reduced time for bioprinting with increased resolution where needed.
Acknowledgments
The authors would like to acknowledge support from the AO Foundation and the German Research Foundation (DFG; project number 326998133 – TRR 225, subprojects B02 and B04).
4. Bioprinting of stem cells
Gregor Skeldon1, 2 and Wenmiao Shu1
1Department of Biomedical Engineering, University of Strathclyde, Glasgow G4 0NW, United Kingdom
2School of Engineering and Physical Sciences, Heriot–Watt University, Edinburgh EH14 4AS, United Kingdom
4.1. Status
Stem cells hold great promise for biomedical research and applications, owing to their strong renewability as a cell source, and potential to differentiate and mature into many cell types in the human body. Roughly divided into differentiated and pluripotent, stem cells have varying self‐renewal and differentiation potential. Through bioprinting, stem cells can be particularly positioned in 3D in relation to other cell types and/or biomaterials. The more well‐defined and easily cultured stem cells, such as mesenchymal, adipose‐derived, and neural were among the first and are the most widely and successfully bioprinted [21]. The successful bioprinting and encapsulation of pluripotent stem cells, both induced and embryonic, has often involved more troubleshooting and can involve bespoke printing technology [22, 23] or materials [24].
4.2. Current and future challenges
Significant hurdles and challenges remain to be overcome before the full potential of stem cell bioprinting can be realized. The major hurdles can largely be summarised as the following three areas:
- 1.Physical printing effects: Stem cells, pluripotent stem cells (PSCs) in particular, are fragile and are sensitive to physical manipulation. When incorporated into bioprinting, cell death can result from the shear stress during printing. Reduction in shear stress, such as by increasing nozzle diameter, reducing print speed, or pressure, can improve cell viability. The bioink used to encapsulate cells can work as a buffer against the shear stress during deposition, but highly viscous materials themselves can cause lethal shear stress to cells.
- 2.Bioink properties: Conventional stem cell culture uses specially coated 2D culture surfaces, often with ECM proteins. This prevents anoikis or adherence‐dependent cell death. When bioprinting, cells must be encapsulated in the bioink, which inherently produces a 3D environment. If no appropriate adherence points are provided, PSCs will undergo anoikis. As well as a support structure for the cells, the bioink must be sufficiently porous to allow nutrient and waste transfer. This must be balanced with maintaining structural integrity of the print: an overly porous ink would produce a weak structure, likely to collapse.
- 3.Biological challenges: Currently, the vast majority of stem cell differentiation protocols are optimized for 2D cultures. Controlled differentiation of 3D cultured stem cells faces issues with diffusion within cell aggregates or spheroids. Additionally, research has shown that 3D differentiation protocols must be elongated from their 2D counterparts for successful cell maturation. Tissue‐level cell density is another problem to overcome in tissue engineering. This would promote cell–cell contact and paracrine signaling, but would cause problems in manufacture due to nozzle blockage, and insufficient nutrient and waste transfer.
4.3. Advances in science and technology to meet challenges
Overcoming the physical limitations of bioprinting using stem cells can be achieved through utilizing various technologies and materials. The first examples of human embryonic stem cell (hESCs) [22] and human inducd pluripotent stem cells (hiPSCs) [25] bioprinting used a bespoke valve‐based deposition system to reduce shear stress. As well as this, laser-induced forward transfer (LIFT) bypasses the need for a nozzle, by directly transferring the cells to the desired substrate [23]. This eliminates the shear stresses present during nozzle‐based deposition.
Selection of an appropriate bioink, the material used to encapsulate stem cells for bioprinting, is crucial. Hydrogels have gained immense favour in bioprinting and bioink research. However, some hydrogels are bioinert with no cell anchorage points. Inclusion of adherent peptides such as RGD motifs within hydrogels promotes stem cell viability and function [25, 26]. Likewise, inclusion of known bioactive gels, such as gelatine [24], Matrigel [27], or a porosity creating gel like carboxymethyl chitosan (CMC) [28], have been found to substantially enhance PSC encapsulation and growth, and led to the first examples of the 3D bioprinted ESCs and hiPSCs.
The 3D differentiation of PSCs by delivering chemical factors throughout printed structures could be improved by porosity of the hydrogel, or incorporating hollow channels, or rudimentary vasculatures. Porosity can be improved by addition of sacrificial materials such as gelatin [24] and CMC [28]. Bioprinting allows production of hollow lumens and vasculatures. It can create high cell density within interior layers, as well as specifically position cells within each layer [29]. The use of these techniques would allow more efficient transfer of factors throughout larger bioprinted structures, and consequently better control of differentiation of the stem cells therein.
The translation of bioprinted stem cell structures to industry or clinical use has different, but significant, roadblocks to overcome [30]. These challenges can be subdivided into scale, quality assurance, and infrastructure. Generating microtissues in a research setting requires roughly 100 million to 1 billion cells. To repair solid tissue or organs through bioprinting, 10–100 billion or more cells may be required [24]. Such an immense increase in scale itself necessitates changes in culture, expansion, and production of bioprinted tissue [24, 27, 29]. The use of PSCs also brings the risk of undifferentiated stem cells generating a teratoma cancer after implantation to a patient; therefore, assurances must be made that all stem cells have sufficiently matured first. Stem cells derived from patients have the potential to create autologous tissue and organ grafts that would be free from immune rejection. For this to be a possibility, however, appropriate facilities would need to exist to: extract; purify; expand; bioprint; and differentiate stem cells into the desired tissue. All this must also be conducted under GMP‐compliant conditions. Currently, such facilities do not exist at scale, but would be essential for practical translation, as well as regulatory approval and possibly oversight.
4.4. Concluding remarks
Stem cells, particularly PSCs, have been heralded for their potential for some time. Despite this, PSC biology is still a relatively new and poorly understood field, which utilizes immature technologies. The 3D fabrication techniques, such as bioprinting, will be essential in realising the potential basic science and therapeutic applications of stem cells, allowing production of more tissue‐like structures. As discussed above, the majority of current differentiation culture protocols were designed and were optimized for 2D culture. The move to 3D culture presents problems that likely require significant alteration of current culture procedures due to the increased system complexity which must be accounted for. New bioprinting technologies in combination with other platforms, such as bioreactors and organs‐on‐a‐chip, can allow more efficient and cost‐effective cell expansion and differentiation compared to standard 2D. As our understanding of the niche in which stem cells reside grows, so too will our capabilities in mimicking that niche in vitro using biofabrication techniques. Alongside better understanding and optimization of the mechanical parameters of bioprinting, it is foreseeable that bioprinting could be the platform to utilize human stem cells to produce artificial solid tissues and organs.
Acknowledgments
Funding support from the EPSRC (Grant No: EP/M506837/1) is greatly acknowledged. G.S. acknowledges the studentship support from Baillie Gifford & Co. and Heriot–Watt University.
5. Large-scale and efficient production of organoids or cell aggregates
Yasuyuki Sakai, Marie Shinohara and Masaki Nishikawa
Department of Chemical Engineering, Graduate School of Engineering, University of Tokyo, Tokyo, Japan
5.1. Status
Because organoids better mimic physiological microstructures of in vivo tissue or organs where heterogenic cell populations are well organized in a hierarchical manner, they are highly expected as advanced microtissue elements for implantable tissue and for in vitro cell-based assays, including disease models. However, as recently reviewed by Schneebergat et al [31], there are many problems with the practical applications of organoids, such as large-scale production, control of maturation in vitro, integration of organoids into much larger tissues having macro-scale tissue structures, etc.
The first important issue to tackle with the use of organoids is their large-scale production. Organoids are usually produced in small-scale tissue culture dishes or plates that are not easily scalable. In addition to just scaling-up of the production, the reduction of the production costs is another serious problem. This is because many expensive growth factors are required in a cocktail and have to be refreshed at each medium change to organize, induce, and maintain organoids derived from stem cells such as induced Pluripotent Stem/Embryonic Stem (iPS/ES) cells or tissue progenitors. To address these issues, we need to integrate basic biological knowledge with various chemical engineering methodologies, including high cell-density bioreactor culture, as well as advanced microtechnologies, such as microfabrication and biofabrication.
5.2. Current and future challenges
Organoid production usually begins with reaggregation of isolated single cell populations in confined geometry, such as microwells in static culture (figure 4). This method is very robust, and we may easily control the sizes of the organoids with a high uniformity. However, it is not readily applicable to larger-scale production. From the stand point of scalability, aggregate suspension bioreactors are the best way, and their applications to iPS cell differentiation towards cardiac, hepatic, or pancreatic lineages have been reported. As opposed to the potential advantages of suspension cultures, control of organization, induction and biological preservation of organoids is much more difficult versus static microwell culture (figure 4). Sometimes initial cell aggregation in dynamic conditions does not succeed. Organization of heterogenic cell populations is usually difficult, presumably because they have lost ECM after their enzymatic isolation. Controlling aggregate sizes is difficult, and excess aggregation through the agglomeration of small organoids causes central necrosis or unintentional development/differentiation. Thus, robust but highly scalable production methods are required to extend the use of organoids.
Figure 4. Current methodologies for efficient production of organoid or cell aggregates; the microwell-based method (a), and the suspension culture-based method (b).
Download figure:
Standard image High-resolution imageAnother important problem common in both microwell-based and suspension culture-based methods is the extensive use of expensive growth factors. In in vivo microenvironments, such growth factors are produced by stromal cells, and immediately and locally act on neighbouring target cells, for example, epithelial cells, at very high concentrations often with the help of ECMs. Although we may partly expect such in vivo-like autonomous mechanisms in organoids, supplementation of growth factors at high concentrations out of physiological ranges is usually necessary to grow and develop organoids in vitro. As alternatives to such expensive growth factors, various small molecules with similar biological activities have been identified and partly used in differentiation protocols of iPS/ES cells. However, these chemicals are often expensive. Ideally, if we reproduce the above-mentioned in vivo autonomous mechanisms in vitro, then, it will definitely lead not only to the enhancement of physiological relevance but also to the reduction in the cost for their large-scale production.
5.3. Advances in science and technology to meet challenges
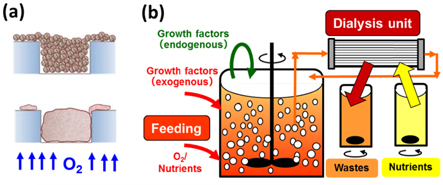
In cases of microwell-based organoid production in static culture, we note its diffusion-limited oxygen supply, and that the use of oxygen-permeable materials such as polydimethylsiloxane (PDMS) for the culture substrates removes this limitation. This enables cells to take aerobic respiration, even at higher inoculum density, leading to remarkably enhanced cell-to-cell organization and ECM production (figure 5). Active ECM production is one of the important keys to the success of heterogenic cell organization into one aggregate. Moreover, we reported that a significant increase in inoculum cell density was achieved, and that it led to remarkable enhancement of per unit area-based productivity of organoids or aggregates, while retaining various advantages of static microwell cultures (figure 4) [32, 33]. In addition, use of oxygen-permeable materials for direct cellular oxygenation can reduce oxygen concentrations to low physiological ranges while keeping aerobic cellular respiration. This may be a very important culture operation to suppress the possible oxidative stresses to immature or progenitor cells, which may not yet have well-developed mechanisms to protect themselves against such oxidative stresses.
Figure 5. How to enhance the efficacy of organoid production from the chemical engineering concepts. An advanced microwell allowing direct oxygenation of cells (a), and incorporation of a dialysis operation in suspension culture (b) for high cell-density production.
Download figure:
Standard image High-resolution imageFor the success of organoid culture in suspension (figure 4), beforehand we sometimes use microwell-based methods to organize isolated single cells in microwell static culture, and then transfer them to dynamic suspension culture. To control excess aggregation, some molecules, such as E-cadherin or other lipid-bearing proteins in culture medium, were shown to inhibit the phenomena [34]. Aggregation of heterogeneous cells in suspension is usually difficult; therefore, some artificial molecules, such as Eudragit (Evonik Nutrition & Care GmbH, Germany), can be used to overcome the low attachability to each other to organize them into one aggregate. Such careful control of aggregation or their development may enable robust and large-scale organoid production with high uniformity, as realized in static microwell methods.
High density culture of aggregates is another important direction for efficient production of large numbers of organoids. Conventional chemical engineering tells us fed-batch dialysis culture may be the ideal culture system [35, 36] (figure 5). Such a system allows an increase in cell density in the cell-culture vessel in which growth factors are added, while the dialysis operation supplies the cells with enough nutrients and removes metabolic waste across the dialysis membranes. This reduces the cost of exogenous growth factors, and may enable full utilization of paracrine or autocrine factors. In addition, this advantage can be further enhanced as the cell density increases. Actually, such a dialysis culture also enabled iPS cell propagation or their hepatic differentiation at very high cell density (over 107 cells cm−3) when we used some reagent suppressing excess cell aggregation (to be published elsewhere). This may lead to further cost reduction of organoid culture in the near future.
5.4. Concluding remarks
Progress in stem cell biology and in vitro culture is opening up new doors to regenerative medicine and better physiological cell-based assays for disease models. However, to bring these advancements into practice, various chemical engineering methodologies and microtechnologies, including biofabrication or microfluidics, have to be fully utilized. Together with such developments, continuous efforts are required to realize better in vivo-like microenvironments at different time lengths and scales in vitro. Unfortunately, at this moment, allowing further in vitro maturation of organoids is still a significant biological challenge; progress remains limited due to insufficient knowledge about tissue development, particularly about in vivo mechanisms of autonomy. Careful study on in vivo mechanisms of autonomy is required with the help of various engineering-based technologies.
6. Strategy for bioprinting of tissue vascular system and tissue assembly
Jinah Jang and Dong-Woo Cho
Pohang University of Science and Technology
6.1. Status
The human vascular system is an organ system that transports oxygen, carbon dioxide, nutrients, hormones, and blood cells, and helps maintain body homeostasis. It is also essential for the prolonged survival and nourishment of engineered tissue constructs that are expected to replace the native human tissues and organs. The lack of control over the organization of the vasculature makes it difficult for organs to function properly. This results in poor assembly of blood vessels after transplantation, and impedes the integration between the construct and the host. In this respect, strategies capable of recapitulating vascular branching in the organs are highly desirable for the engineering of large-volume tissues.
Cells (e.g. parenchymal, stromal, and supporting cells) reside in the surrounding microenvironment that consists of various ECMs, as well as with external stimuli and soluble factors, enabling them to perform specific functions. For instance, properly positioned stromal and supporting cells help synthesize and remodel the ECM, and the secretion of specific cytokines contributes to the creation of a tissue-specific microenvironment. Therefore, proper biofabrication strategies can facilitate the pre-positioning of cells at the desired locations, inducing self-assembly of tissue architecture and supporting tissue mimicry.
A multicellular 3D bioprinting approach for vascularization is emerging as a highly promising strategy [37]. It enables the embedding of perfusable channels, as well as the spatial positioning of vascular cells (e.g. endothelial progenitor cells (EPCs), human umbilical vein endothelial cells (HUVECs), induced pluripotent stem-cell-derived endothelial cells (iPSC-ECs), and smooth muscle cells) in the engineered construct. For 3D printing of an intravascular network, the size of the vasculature is usually less than 500 μm, close to the microvasculature range (50–500 μm). The distance between the tissue and the vessel should be around 100–200 μm and is a critical issue: a distance larger than 200 μm could impede blood circulation or molecular diffusion in the engineered tissues after transplantation.
To achieve such specific design criteria, many strategies have been attempted: (1) micropatterning of vascular cells for inducing tissue assembly [38, 39], (2) sacrificial channel fabrication and moulding [40], (3) direct printing of perfusable vessel-like structures [41], and (4) patterning of perfusable structures with specific architectures [37]. These recent advancements in biofabrication have shown remarkable abilities to facilitate thick and large tissue reconstruction, shortened time for anastomosis, and delivery of high numbers of cells.
Although numerous biofabrication strategies have been successfully applied to mimic sophisticated human tissues, complex and multistep fabrication processes restrict the hierarchical design for higher-order tissue modelling. In addition, this complicated process increases the time required for construction and affects the viability and functionality of the printed cells. Thus, modularisation techniques have been attempted to fabricate human-scale tissue constructs more appropriately.
Modules (e.g. cell aggregates, laminar cell sheets, biomolecules) can induce self-assembly by virtue of cellular sorting or tissue fusion processes, form the systemic level, and evolve into the quiescent state, indicating a structurally and energetically stable state [42]. The physical assembly of each module or polymeric framework as the LEGO® block may represent an additional solution. However, it usually requires delicate assembly processes, resulting in operator-related variability and poor tissue morphogenesis. Therefore, cellular/physical assembly performed by computer-assisted fabrication methods is very much required.
6.2. Current and future challenges
The intricate nature of intra- or inter-tissue connections complicates the process of natural tissue formation. Printing of perfusable channels or blood vessel analogues has enabled us to recapitulate the tube-like structure of endothelial cells and exploit their ability to spontaneously form a lumen by supporting the niche microenvironment [41]. Along with the spatial pre-positioning of cells and the use of bioinks, the application of external stimuli (e.g. shear/cyclic stress, pulsatile force) may represent an additional factor for enhancing tissue maturation [43, 44]. In particular, shear stress is crucial for the secretion of growth factors (e.g. platelet-derived growth factor and transforming growth factor-beta 1) and the activation of the associated molecular signals leading to ECM deposition (e.g. collagen, elastin). However, the creation of the physical connection between the structure and the circulation system (e.g. syringe pump, peristaltic pump) is a critical issue. In addition, the end-to-end anastomosis between the structure and the native vessel is a remarkably tricky process.
The tissue-specific microenvironment, specifically the surrounding matrix, can biochemically induce tissue morphogenesis. Recent studies applying tissue-specific ECM-based bioink (e.g. decellularized ECM derived from fat, cartilage, heart, liver [38, 45, 46]) showed a striking enhancement of numerous biological processes in embedded cells, including cell proliferation, differentiation, angiogenesis, and tissue formation. Although these bioinks have proved the possibility of promoting tissue maturation, technical improvements are required to more tightly control the structural integrity by regulating physicochemical characteristics.
6.3. Advances in science and technology to meet challenges
Bioprinting-based modular tissue assembly can facilitate the highly defined and controllable construction of large-volume tissues. The modules are largely categorised into three types: cellular, ECM, and structural modules, based on the simplification of complicated human tissues, and reflect the assembly of these functional units in a layer-by-layer approach. Various cellular and ECM modules, such as endothelial and stromal cells (e.g. fibroblasts, pericytes, MSCs), cell aggregates, spheroids, hydrogels (e.g. synthetic, natural, hybrid biomaterials), and bioinks, a formulation of cells suitable for processing by biofabrication, have been specifically positioned in the constructs. The design of the modular assembly varies depending on the vascularization strategy (e.g. sprouting-, self-assembly-, sacrificial channel-based approaches) and the geometry of vessels (e.g. branching angle, branching frequency, tortuosity). In addition, advanced bioinks enable rapid lumen formation by providing tissue favourable binding sites to the endothelial cells. Various approaches, including vascular tissue-derived ECM bioink and coaxial nozzle-based printing [41], and photodegradable materials containing the integrin-binding RGDS peptide sequence (H-Arg-Gly-Asp-Ser-OH) with the projection-based microstereolithography technique have been explored for direct lumen formation and endothelialisation [40]. Further examination and application of these bioinks is required to build tissue constructs. Similarly to cellular-ECM modules, structural modules (e.g. polymeric scaffolds) can also be considered as building blocks, indicating that the structural, cellular, ECM, interfacing, and fixation characteristics are critical factors for a higher level of tissue integration [42].
6.4. Concluding remarks
Current advances in biofabrication have shown remarkable capabilities to recreate human vasculatures and large organs. Various attempts have been made to promote modular tissue formation as well as vessel formation, including early anastomosis between microvessels in the construct, blood circulation, and tissue maturation after the transplantation. Specifically, automated bioprinting technologies can collectively provide necessary cells, functions, and microenvironmental cues for achieving large-volume tissue construction. Although the challenges facing this strategy are immense, achievements may lead to clinical applications for providing advanced therapeutic methods, understanding disease mechanisms, and engineering of microtissue models.
Acknowledgments
This research was supported by the National Research Foundation of Korea (NRF) grant (No. 2019R1A3A3005437, 2015R1A6A3A04059015), and the 'ICT Consilience Creative Program' (IITP-R0346-16-1007) supervised by the IITP (Institute for Information & Communications Technology Promotion).
7. 3D-printed biohybrid tissues as in vitro biological models for disease study
Minghao Nie and Shoji Takeuchi
The University of Tokyo
7.1. Status
In vitro reconstructed tissues need precisely controlled 3D arrangements of cells and ECMs to correctly predict drug responses for disease study. By blending 3D printing with the technical know-how of biomaterial handling from the field of tissue engineering, 3D bioprinting holds the potential to create better disease models by enabling rapid fabrication of tissues with accurate spatial arrangement of cells [47].
Within decades of development, bioprinting has transformed itself from science fiction to reality by breaking through two major hurdles: cell-viability loss, and the inability to scale-up. First, cells suffer viability loss since printing conditions are not always optimal for cell survival; the shear forces necessary for the ejection of cells/ECM, as well as pH/temperature change during printing, will cause damage to cell membranes, and therefore leads to cell death. To maintain cell viability, optimized biomaterials and printing conditions have been determined for various printing modalities, such as inkjet-based and extrusion-based. Biomaterials, which can buffer pH change and rapidly crosslink during printing, have been developed to incorporate cells. Improved post-printing cell viability is achieved by optimizing the designs of the printheads to reduce printing shear stress, and sterile bioprinter housings to prevent contamination. Second, with the prevalence of extrusion-based printing methods, the rapid fabrication of millimeter-scale constructs with precise 3D spatial arrangements of cells has been made possible. These capabilities have drastically boosted clinical R&D by shortening the sample preparation time for drug screening.
7.2. Current and future challenges
Despite current achievements enabling rapid printing of cell-laden hydrogel constructs with high cell viability, many functions that are the hallmarks of in vivo tissue are not yet successfully replicated. These functions are essential for the evaluation of drug responses in many tissues, such as the multiple-layered barrier function of the vascularized skin or the large force-to-displacement transduction function of skeletal muscle/cardiac tissue.
First, the multiple-layered barrier functions to control the transdermal delivery of external-use drugs through the epidermis of the applied location all the way to the capillary vessels beneath the dermis. For example, the evaluation of cosmetics includes toxicity-induced diseases and requires measuring the amount of drug-containing toxic compounds that penetrate the skin into the blood stream. To fulfill such requirements, in vitro reconstructed skin equivalents shall be composed of a well-differentiated epidermis, an epidermis–-dermis barrier with a firmly deposited basal membrane, fibroblast-populated dermis, and an endothelial barrier consisting of a layer of endothelial cells with tight junctions. Current technology for the fabrication of a skin equivalent benefits from the contraction of a fibroblast-populated collagen lattice (FPCL) [48]. Based on such techniques, keratinocytes can be subsequently seeded on top of the contracted FPCL to form an epidermis layer. However, current technology has not achieved the incorporation of capillary vessels into the FPCL; FPCL contraction leads to the collapse of perfusable lumens and the detachment of FPCLs to the injection needles/nozzles. For the reconstruction of skin equivalents with well-established transdermal barrier functions, techniques that can support the stable perfusion of FPCLs are strongly required.
Second, the large force-to-displacement transduction function is the trademark of skeletal muscle/cardiac tissue. Analyzing the displacement actuation provides the opportunity to directly visualize scoring on the force generated by skeletal muscle in response to drugs. Even though tiny muscle tissues with highly aligned actin filaments have been fabricated in vitro, the contractile motion of these fabricated tissues is generally small since they lack the incorporation of tendon/skeletal parts, which have the potential to amplify their motion. To establish the large force-to-displacement transduction function of skeletal muscle/cardiac tissues, techniques such as 3D printing/bioprinting may allow firm assembly of muscle tissues with cantilever/joint-type skeletal/tendon constructs. In addition, the incorporation of neuron-muscular junctions into the muscle tissue is also important since it allows the modelling of neuron-related muscle disease.
7.3. Advances in science and technology to meet challenges
We believe that 3D-printed biohybrid tissues, which combine 3D-printed non-living parts (such as polymers/electrodes) with biofabricated/bioprinted cellular parts, could potentially solve the above-mentioned challenges. These biohybrid tissues take advantage of the specifically designed mechanical/electrical properties of non-living (inert) parts and connect these parts firmly with the biofabricated tissues so that the chemical, mechanical, and electrical stimulation can be correctly applied to test specific tissue functions.
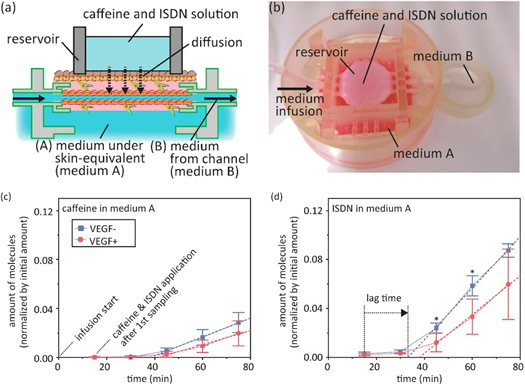
In terms of skin fabrication, we have demonstrated the potential of our biohybrid approach for the reconstruction of the multiple-layered barrier function of in vitro skin equivalents. These systems aim to create an in vitro model for the transdermal delivery of external-use drugs/cosmetics. Using these tools, we have fabricated skin tissues in 3D-printed tissue housing with sacrificial nylon wires threaded through bulky tissues to create perfusable vascular networks (figure 6) [49, 50]. The detailed fabrication process is as follows. First, a piece of skin equivalent is fabricated by pouring a fibroblast/collagen mixture into a 3D-printed housing followed by the subsequent seeding of keratinocytes; after the formation of the skin equivalent, the fabricated dermis can firmly anchor to the side wall of a 3D-printed housing, even after drastic contraction of the dermis tissue. Next, a lumen is formed by pulling out the sacrificial nylon wire followed by the subsequent seeding of HUVECs. This fabricated skin equivalent could withstand pressures required for sample perfusion during drug screening. To investigate the influence of vascular perfusion on percutaneous absorption, we have monitored the permeation of test molecules, caffeine and ISDN (isosorbide dinitrate), through the skin equivalent during the perfusion of Dulbecco's Modified Eagle Medium (DMEM) in the vascular channels; the normalized permeated amount of ISDN was three- to fourfold higher than that of caffeine [49]. The differences in permeability between caffeine and ISDN, which are consistent with previous studies, have shown the applicability of our 3D-printed hybrid skin as a promising in vitro model for skin disease therapy development. In the future, we plan to replace the moulding methods previously adopted for dermis/epidermis fabrication with robotic-assisted bioprinting methods to create more accurate cell/ECM distribution.
Figure 6. A skin equivalent integrated with perfusable vascular channels for drug permeability testing. (a), (b) A schematic and photograph of the experimental setup. (c), (d) Amounts of caffeine permeating the medium beneath the skin equivalents (medium A, B). Copyright: reproduced with permission from [49], copyright 2017 Elsevier Ltd.
Download figure:
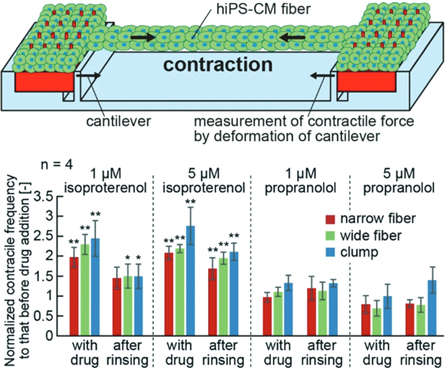
Standard image High-resolution imageIn terms of muscle fabrication, we reconstructed the large force-to-displacement transduction function of biohybrid skeletal muscle/cardiac tissue by incorporating anchoring structures. In detail, we 3D-printed pillar arrays (e.g. with electrodes patterned using photolithography) using SLA and micromachining. Then, cells were dispensed onto the prefabricated 3D parts; upon culture, cells condensed and grabbed the anchoring structures, thus forming tight mechanical connections between a non-living (skeletal construct) and living parts (muscle tissue). Using this strategy, we have successfully fabricated muscular tissues, and were able to transform muscular contraction to mechanical motion for the modelling of muscular systems (figure 7) [51–53]. To demonstrate drug reactivity, we applied isoproterenol and propranolol to the biofabricated tissue, and we were able to detect an increase in peak-to-peak (p–p) contractile force after the addition of isoproterenol, and a decrease in p–p contractile force after the addition of propranolol [51]. In the future, we will attempt to extend our biohybrid strategy to multiprinthead bioprinters [54] to produce the biohybrid skeletal muscle/cardiac tissue with high-throughput in the future. In addition, we will attempt to incorporate nano-scale details into the 3D-printed parts using high-resolution 3D printing technology so that the biohybrid microtissues can better model the in vivo muscle tissue [55].
Figure 7. A schematic illustration (top) of the contractile force measurements of the human induced pluripotent stem cell-derived cardiomyocytes (hiPS-CM) fibres. The contractile force was estimated from the cantilever deformation. The graphs (bottom) show the contractile frequency of the hiPS-CM fibres and clumps 30 min after the addition or removal of isoproterenol and propranolol. Copyright: reproduced with permission from [51], published by The Royal Society of Chemistry.
Download figure:
Standard image High-resolution image7.4. Concluding
Bioprinting is a field that is coming-of-age, with the early achievements in the fabrication of cellular constructs, with tissue and disease model value. To further transform the technology for disease modelling, challenges—such as the establishment of perfusable multilayered skin barrier function and the realization of the large force-to-displacement transduction function of muscular tissue—have yet to be met but appear within reach. We propose to overcome these challenges by creating 3D-printed biohybrid tissues to which chemical, mechanical, or electrical stimulation can be applied to create disease models of single/multi tissue systems. Our strategy has the potential to be fully automated, and therefore can be adapted for the mass production of functional biofabricated tissues for disease study.
Acknowledgments
We would like to thank all members of the BioHybrid System Lab, The University of Tokyo for their help and support.
8. 3D bioprinting for organ-on-a-chip development
Ostrovidov S and Khademhosseini A
8.1. Status
The human body is composed of tissues that interact with each other to form complex organs and systems. To mimic the biological relationships in vitro, microfluidic systems and perfusion chambers have been integrated to simulate in vivo microenvironments. These systems, known as organs-on-a-chip, facilitate the study of cell and tissue level interactions by incorporating relevant cell types, fluid flows, and biomolecules. These platforms mimic physiological conditions better than conventional tissue culture models; their ability to emulate human physiology makes them particularly useful for drug development as they provide better data on drug efficacy and side effects [56]. Biomimetic organ-on a-chip systems replicating in vivo behaviour have been developed for the liver, kidney, heart, gut, breast, and blood vessels. The connection of different modules to form multiorgan-on-a-chip devices facilitates the investigation of complex physiological conditions. The ultimate goal is to link several chips to create a human-on-a-chip platform for drug testing and disease studies [57]. The complementary technology of 3D bioprinting allows precise deposition of cell-laden matrices to fabricate biological structures. Due to its suitability for translation to high-throughput fabrication and automation, the combination of 3D bioprinting with microfluidics allows the development of the next generation of organ-on-a-chip platforms. Lee and Cho reported a one-step fabrication method for an organ-on-a-chip by 3D printing polycaprolactone for the chip structure, in addition to gelatin, collagen, liver cells, and endothelial cells for the biological model. Another study by Zhang et al fabricated an endothelialized myocardium by printing endothelial cells in a composite bioink. After endothelial cells migrated towards the periphery of the printed fibres, cardiomyocytes were seeded on the construct. After incubation, the myocardial tissue demonstrated synchronous beating and was used to perform drug toxicity testing [58]. As the field of 3D bioprinting continues to advance, current research focuses on integrating research techniques (e.g. bioprinting and microfluidics) to print heterogeneous structures, multimaterial bioinks, and stimuli-responsive bioinks and create complex organ structures [59] (figure 8).
Figure 8. Multimaterial bioprinting of 3D constructs. (A), (B) Bioprinting of dual‐ and triple‐layered cuboid blocks. (C)–(E) Bioprinting of blood‐vessel‐like structures (transverse plane) containing dual, triple, and quadruple materials. (F) Bioprinting of a pyramid containing seven layers of different bioinks. (G), (H) Bioprinting of three‐ and ten‐layered blocks with continuous segments of seven different bioinks. (I) Bioprinting of human organ‐like constructs from multiple bioinks, including brain, lung, heart, liver, kidneys, pancreas, stomach, small/large intestines, bladder, and prostate. The organ‐like constructs were individually printed, photographed, and stitched together in the same image at relative locations as those in the human body. (J)–(N) A side view of selected organ‐like constructs indicating their 3D nature: (J) brain, (K) lung vasculature, (L) kidney, (M) left atrium of heart, (N) bladder/prostate. The organ‐like structures were not printed to scale to each other. From Liu et al 2017 [60], ©2016 John Willey and Sons.
Download figure:
Standard image High-resolution image8.2. Current and future challenges
When building an organ-on-a-chip system, the selection of the targeted organs and cell types is important. While human cells are a relevant choice, achieving in vivo-like functionality of primary and immortalized cell lines, including stem cells, in vitro is challenging. Since interactions between organs are important in drug testing, multiple tissues must be integrated in the organ-on-a-chip. Therefore, a universal culture medium with adequate growth factors that supports the growth and differentiation of different tissues is required [61]. This method takes into consideration the whole cells and tries to find a balance between the different components required by each cell type. One method often used involves mixing in equal parts the standard culture medium required by each cell type to obtain a common culture medium. Another approach is to chemically define a culture medium [62]. This method is developed to obtain a culture medium free of any components of animal origin that is optimized to improve the proliferation, differentiation, and maturation of human cells for clinical applications. Moreover, real-time monitoring of the tissues is needed. Multiple sensors can be integrated into the microchip to evaluate specific biomarkers and functional parameters for each tissue (figure 9) [63]. The integration of imaging capabilities into the microdevice will facilitate automation by collecting feedback to control processing and harvesting of experimental samples. In terms of 3D printing, the engineering of different tissues requires the use of bioinks adapted to each tissue's development. Such bioinks must be able to maintain cells in homogeneous suspension over time, have good printability, and mimic the native ECM of each cell type to support optimal cell proliferation and differentiation. A recurrent challenge in tissue engineering is vascularization. Bioprinting of thick tissue remains challenging, and requires the support of a vascularization network. To approach the structural complexity of organs, multimaterial bioinks with similar viscosities should be developed to allow spatial deposition of different cell types. The dynamic change in the ECM composition seen in vivo may be mimicked via microfluidics (through the delivery of growth factors and signaling molecules), while the use of stimuli-responsive bioinks may reproduce dynamic events and deliver molecules locally. Research on different materials for microfluidic device fabrication is also needed. Although PDMS is most commonly used for microdevice fabrication due to its biocompatibility, optical clarity, and gas permeability, it may not be suitable for all situations as it can absorb small organic molecules, including drugs.
8.3. Advances in science and technology to meet challenges
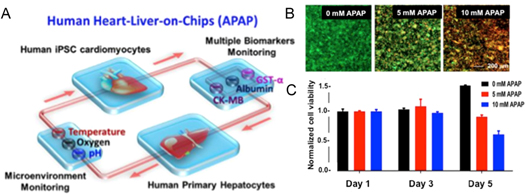
Human cells have been used for bioprinting to develop platforms that better mimic human physiology. Human stem cells, with their high potency and ability to proliferate and differentiate into multiple cell types, are well suited for organ fabrication. However, new bioinks supporting their proliferation and differentiation must be developed. The use of human-induced pluripotent stem cells (iPSCs) will allow the fabrication of organs-on-a-chip for personalized medicine in model disease and drug testing. Additionally, multimaterial bioinks have been developed with the ability to support a variety of cell types and spatially segregate formation of distinct tissue structures [60]. However, more multimaterials like these are needed to facilitate printing a wider variety of cells. Many efforts have also been made to bioprint blood vasculature. Coaxial bioprinting has been developed for the fabrication of tubular constructs to recreate vessel structures. Recently, Pi et al showed one-step fabrication of multilayered tubular tissues with cellular heterogeneity using a multichannel coaxial extrusion system [64]. Digital micromirror device (DMD) bioprinting combined with computed tomography has also yielded promising results in vascular fabrication. These efforts should be pursued further to develop high-throughput fabrication methods with the ability to bioprint thick (>1 mm), vascularized, heterogeneous tissues for implementation into organ-on-a-chip systems. Recently, Bhise et al fabricated a liver-on-a-chip platform with a bioprinted dot array of hepatic spheroids encapsulated in GelMA hydrogel. They monitored hepatic function for one month, and used the platform to evaluate the toxicity of acetaminophen on liver cells [65]. In another study, Skardal et al bioprinted liver, heart, and lung organoids to develop a multiorgan-on-a-chip platform to investigate the interactions between organs and the individual and collective response towards drugs and toxins [57]. Furthermore, they developed biosensors to provide real-time data acquisition on the microfluidic platform.
Figure 9. An integrated, automated multiorgan-on-a-chip platform with sensing capability. (A) A schematic of a biomimetic human heart- and liver-on-a-chip. (B) An integrated primary hepatic and iPSC-cardiac dual-organoid platform, live/dead staining of the hepatic organoids after 0, 5, 10 mM acetaminophen (APAP) treatment for 120 h. (C) Normalized cell viability in the presence of APAP from day 1 to day 5. Modified from Zhang et al 2017 [63].
Download figure:
Standard image High-resolution image8.4. Concluding Remarks
Current development in 3D bioprinting has focused on the fabrication of tissues with greater complexity and a better ability to mimic in vivo behaviour. To achieve increased ex vivo similarity, size, and complexity, efforts have been concentrated on the engineering of thick tissues and the integration of vasculature. Furthermore, the use of human stem cells demands improved matrices to support their growth and differentiation. In the future, it may be possible to develop in vivo-like organs in vitro to revolutionize modern medicine and healthcare by developing organ-on-a-chip platforms used in personalized drug screening, drug development, and improving drug safety.
Acknowledgments
The authors acknowledge Peyton Tebon for helpful comments.
9. Biomanufacturing of multicellular engineered living systems
Roger D Kamm
Massachusetts Institute of Technology
9.1. Status
Over the last decade, several key advances have been made with important implications for the development of multicellular engineered living systems (M-CELS). First, the Yamanaka group in Japan demonstrated that induced pluripotent cells could be produced from human dermal fibroblasts [66] using a newly developed protocol. While the experimental procedures have since been refined and extended, it is now widely accepted that a pluripotent cell population can be obtained from fibroblasts of any subject. Second, in 2011, the first reports appeared describing the methods that could be employed to produce an organ-like cell assembly from a cluster of pluripotent cells to produce an optic cup [67]. Many others have appeared since, notably organoids for the brain [68] and hindgut [69]. And lastly, the continued development of microfluidic technology based on soft lithographic methods, has fostered the growth of microphysiological systems; M-CELS that exhibit some aspects of organ form and function have demonstrated potential for disease models, and show promise for drug screening (figure 10). Numerous organ systems have now been reported in the literature and the number continues to grow, as does their ability to replicate real human organ function. The combination of these three technologies has led to the recent optimism among many, that human organ systems can be produced with sufficient realism and consistency that opens the door to various applications, but importantly, as a new and valuable tool for the pharma and biotech industries. Indeed, most, if not all, major pharma companies now have vigorous activities in these areas.
At present, there are essentially two methods used to fabricate M-CELS [70]. In one method—'top-down engineering'—cells are seeded onto or into substrates, in precisely the format needed to perform their function. Simple monolayers fall into this category, as well as cells widely dispersed in a natural or synthetic matrix, that are not required to interact. The second method takes advantage of the natural ability of cells to differentiate and self-organize; one might call this 'emergent engineering'. The first of these follows well-established procedures, and is well suited to bioprinting. The latter is much less established or understood, and the applicability of bioprinting depends on the type of system being fabricated. Issues of cell density, cell viability, matrix composition and density, and variety of cell types needed all come into play, and in the end, one needs to rely on the intrinsic capability of the cells to modulate their phenotype, through interactions with their neighbouring cells and matrix, in order to gain the desired form and function. In the extreme, the growth of organoids from aggregates of pluripotent cells requires no printing at all; the cells do the work themselves, developing the minute structures characteristic of a particular organ through their inherent, 'hard-wired' programs. Moreover, these same intrinsic competencies enable the 'manufactured product' not only to self-assemble, but also to self-repair, adapt to changing conditions, and even potentially to self-replicate.
Figure 10. A schematic showing the process for producing M-CELS for various applications.
Download figure:
Standard image High-resolution image9.2. Current and future challenges
To develop M-CELS that meet the rigorous requirements of industrial application, or even the need for repeatability in the research setting, a new approach is needed in biofabrication. Driven by the recognition that the manufacture of biological systems is fundamentally different from that of abiotic systems, entirely new approaches to design, manufacture and quality control are needed. These differences represent, at the same time, barriers to progress and opportunities. One barrier is that much of the developed practice based on non-biological systems—including virtually all the manufactured items now produced—is largely inappropriate for M-CELS. All current design and manufacturing principles are based on the fact that the interactions between the various components of a system can be predicted based on known physical principles. In biological systems, the components (cells and molecules) interact in ways that we have still only begun to appreciate. Take, for example, the ability of a collection of muscle cells to self-assemble into a muscle bundle and produce collective contractions [71], or the ability of a population of endothelial cells to self-organize into a 3D network with perfusable lumens [72]. While even these relatively primitive processes remain beyond the reach of our understanding, they also present an opportunity. In creating a muscle, one need not place each myocyte in the precise location desired in the final muscle 'actuator', but simply place them in close proximity that enables them to interact naturally and to self-organize. Numerous examples can be found in which such self-assembly occurs and makes possible the creation of organ-like systems. One enormous challenge is to establish the fundamental understanding necessary to take a brain organoid, for example, and induce it to grow an appropriate vasculature, or to introduce functional inputs and outputs in order to take advantage of the brain's unique information processing capabilities.
Other features of natural biological systems—self-repair, adaptation, even self-replication—all pose opportunities, if advanced through a reasoned approach, keeping in mind the potentially important ethical implications of such capabilities.
9.3. Advances in science and technology to meet challenges
Bioprinting represents but one of many approaches that, when further developed, can play an important role in the manufacture of M-CELS. Drawing upon the self-organizing capabilities of living cells, it has already proven sufficient in a number of studies, when growing a particular biological component, to place the various cells in sufficiently close proximity to each other in an appropriate matrix material or on a suitable substrate and let them 'do their own thing.' But tissues are dense cellular structures, and current bioprinting methods are limited in their ability to print at the high cell concentrations needed, or with the spatial resolution necessary for the smallest biological structures [73]. In going to finer and finer resolution, the shear forces on the cells being printed will also increase, raising concerns about the potential for cell damage, or at least activation due to the mechanical stimulus.
Perhaps the more important need is for a reliable computational approach to predict the structure and performance of the resultant system. While engineered systems are relatively straightforward, especially those containing simple structures such as monolayers, more complex tissue arrangements mandate a more rigorous and comprehensive understanding, which will subsequently lead to modelling capabilities.
9.4. Concluding remarks
There is little doubt that bioprinting will prove to be a useful tool in the fabrication of M-CELS, but its range of applicability will depend on several factors and the ability to surmount the challenges mentioned above. There will need to be a fundamental change in the way that we address the fabrication of complex biological systems, and the ability for self-assembly needs to be embraced and understood. Biological variability also needs to be recognized and reduced to the extent possible. Finally, much work is needed before the full potential of organoid systems can be fully realized. And when it is, the role for bioprinting needs to be re-examined. Perhaps it comes in when we start to address the need to interact with or interrogate the organ, but many questions remain, and the field is currently wide open and ripe for important advances.
Acknowledgments
Support from the National Science Foundation for the Science and Technology Center on Emergent Behaviors of Integrated Cellular Systems (CBET- 0939511) is greatly appreciated.
10. Bioprinting in space: pioneering new frontiers
Vladimir Mironov1 and Lorenzo Moroni2
13D Bioprinting Solutions, Moscow, Russia and the Institute for Regenerative Medicine, Sechenov Moscow Medical University, Moscow, Russia
2MERLN Institute for Technology-Inspired Regenerative Medicine, Complex Tissue Regeneration Department, Maastricht University, Maastricht, The Netherlands
10.1. Status
Three-dimensional bioprinting could be defined as a robotic biofabrication of 3D functional tissue and organ constructs from living cells and biomaterials according to a digital model. Several research groups and companies in the USA, European Union, Russia, and China are already actively preparing the necessary research infrastructure and biofabrication devices, such as bioassemblers and bioprinters, for 3D bioprinting in space. Bioassemblers could be defined as devices that enable the bioassembly of 3D tissue and organ constructs which do not require traditional layer-by-layer additive biofabrication, and could be based on novel principles of formative biofabrication (see below) and programmed self-assembly. In this context several logical questions are arising:
- (i)What is a rationale for performing bioprinting experiments in space?
- (ii)What are the potential advantages of bioprinting in space compared with more traditional tissue engineering approaches?
- (iii)How 3D bioprinting in space will advance biofabrication and bioprinting technologies.
- (iv)How bioprinting technology could be used to enable further human deep space exploration, including establishing long-term sustainable human planetary settlements.
Extrusion-based 3D bioprinting is the most popular and broadly used variant of bioprinting technology on Earth. Thus, it is not a big surprise that the first 3D bioprinter tested under the conditions of microgravity in space during a parabolic rocket fly was an extrusion-type bioprinter. Several USA companies are actively working on the development of certified space 3D bioprinters of the extrusion type for performing bioprinting experiments at The International Space Station (figure 11). The main challenge of extrusion-based 3D bioprinting using bioinks is finding the optimal conditions between the printability and fidelity of bioprinted constructs and their biocompatibility. Viscous bioinks have bioprintability with a high level of fidelity, but their biocompatibility and permissiveness for post-printed sprouting angiogenesis are usually compromised. Three-dimensional bioprinting under the conditions of microgravity in space allows the use of more biocompatible and usually less viscous bioink, thus enabling bioprinting of geometrically more complex tissues and organ constructs with large empty space, channels, and voids. Therefore, extrusion-based bioprinting under the conditions of microgravity in space have certain advantages compared with bioprinting under the conditions of gravity on Earth. Bioprinted in space tissue models can be used to study the physiology of tissues and organs exposed to space conditions, such as microgravity and radiation. This will be important in understanding the biological effects of the space environment for long-term manned missions [74–80].
Figure 11. An extrusion-type space 3D bioprinter 'Allevi ZeroG' (according to Allevi, USA with permission).
Download figure:
Standard image High-resolution image10.2. Current and future challenges
Magnetic levitation is another emerging space bioprinting technology. The American company n3D Biosciences, Inc. already performed magnetic levitational experiments at The International Space Station in 2017. One of the objectives of the project 'Magnetic 3D Cell Culture for Biological Research in Microgravity' was to use magnetized cells and tools to make it easier to handle cells and cultures, to improve the reproducibility of experiments, and to determine whether biological events in these cultures result from gravity or substrate attachment. However, magnetic levitational experiments have been performed without employing the specially designed magnetic 3D bioprinter. The Russian company 3D Bioprinting Solutions plans to use a label-free magnetic levitational bioassembly of tissue engineered constructs or so-called 'formative biofabrication', where a magnetic field will be used as a sort of temporal support or 'scaffold'. A magnetic space bioprinter 'Organ.aut' has been already designed, produced, and certified for performing bioprinting experiments at The Russian segment of The International Space Station in October 2018 (figure 12). Implementation of magnetic levitation under the conditions of gravity on Earth requires using relatively toxic paramagnetic medium, including, for example, gadolinium. Microgravity conditions in space will enable magnetic levitation using a 100 times lower concentration of gadolinium. It has been already demonstrated in our recent experiments at The European High Magnet Field Laboratory in Nijmegen, The Netherlands, that a high magnetic field also enables magnetic levitational bioassembly at low concentrations of gadolinium. However, this requires a specially designed supermagnet, which consumes a lot of energy. Thus, the magnetic levitational bioassembly under the conditions of microgravity in space is an attractive alternative for advancing, rapidly emerging, scaffold-free, label-free, and nozzle-free formative biofabrication or magnetic 3D bioprinting using a non-toxic concentration of a paramagnetic medium. It will allow a rapid biofabrication of tissue engineered constructs directly in microgravity in space. Furthermore, the combination of magnetic and acoustic levitation has the potential to enable rapid biofabrication in space of 3D tissue and organ constructs of complex geometrical forms according to a desirable digital model using magneto-acoustic bioprinters, which are yet to be developed.
Figure 12. The design (top) and implementation (bottom) of the magnetic space 3D bioprinter Organ.aut, already certified for performing magnetic levitational bioassembly experiments at The International Space Station in 2018 (according to 3D Bioprinting Solutions, Russia).
Download figure:
Standard image High-resolution image10.3. Advances in science and technology to meet challenges
Bioprinting in space is subject to strict regulations and approval by correspondent national space agencies. The so-called 'flyability' of bioprinters or permission and approval to use 3D bioprinters in space stations requires a complex elaborated certification procedure. The space bioprinters and consumables used during the bioprinting process, such as bioinks, cells, and cell-culture media, must be safe and not bring any potential additional risks to the health of astronauts, and in the future to space travelers or tourists. Maximal automation and user friendliness will guarantee the effective performance of bioprinters in space, and will not require sending of additional specially trained experts in bioprinting. Astronauts must be well trained on Earth before space travel and able to effectively operate 3D bioprinters in the absence of experts in bioprinting on board space stations or in planetary settlements. The limit of space at the already existing international and developing national space stations also demands compact bioprinters. The limit of energy is another restricting factor, forcing the development of maximally compact and relatively small space bioprinters. Thus, the ideal space bioprinters must be safe, maximally automated, compact, and user friendly.
Space radiation is considered as a main impediment on the way to advancing human space exploration and, especially, for deep space travel and establishment of sustainable planetary settlements. A Van Allen magnetic belt protects humans on Earth and astronauts at the low orbit from the negative effects of space radiation. However, in the case of deep space travel and planetary settlement, humans will be subjected to the much stronger negative effects of space radiation. Bioprinted human tissue constructs may be used as some sort of sentinels or radiation biosensors for biomonitoring of the potential negative effects of space radiation in deep space, for example, on the Moon and Mars, on bioprinted living radiation-sensitive human tissues. It is an already well-established fact that the radiosensitivity of human tissues and organs is connected with their proliferation. In other words, highly proliferative tissues are more radiosensitive. Thus, stem cells, bone marrow, and intestinal epithelium, as well as reproductive organs such as ovaries and testes, are primary candidates for bioprinted human tissue sentinels. The bioprinted human cells, tissues, and organs could be used as radiation biosensors or sentinels, either in frozen or in living conditions.
10.4. Concluding remarks
Bioprinting in space is one of the novel promising and perspective research directions in the rapidly emerging field of biofabrication. There are several advantages of bioprinting in space. First, under the conditions of microgravity, it is possible to bioprint constructs employing more fluidic and, thus, more biocompatible bioinks. Second, microgravity conditions enable 3D bioprinting of tissue and organ constructs of more complex geometries with voids, cavities, and tunnels. Third, novel scaffold-free, label-free, and nozzle-free magnetic levitational bioassembly technology or formative biofabrication could be implemented under the conditions of microgravity. The ideal space bioprinters must be safe, automated, compact, and user friendly. They could be based on different variants of bioprinting technologies, including extrusion- and magnetic-based bioprinting, which have already been explored in space. Thus, there are no doubts that systematical exploration of 3D bioprinting in space will advance biofabrication and bioprinting technology, and could be considered as new exciting research frontiers.
Acknowledgments
The authors thank the Russian Space Agency for support.
Disclosure
Vladimir Mironov is a Chief Scientific Officer of the company 3D Bioprinting Solutions.
11. Bioprinting technologies
Ibrahim T Ozbolat
11.1. Status
Bioprinting can be defined as the simultaneous positioning of biomaterials and living cells in a prescribed layer‐by‐layer stacking organization to fabricate engineered tissues and organs. Depending on their governing physical mechanisms and deposition means, bioprinting technologies can be classified into three major modalities: extrusion‐based bioprinting (EBB), droplet‐based bioprinting (DBB), and laser‐based bioprinting (LBB) (figure 13) [81]. EBB utilizes extrusion force by means of pneumatic‐, mechanical‐ (screw‐or plunge‐driven), or solenoid‐based systems in order to deposit bioinks in the form of continuous filaments. DBB, on the other hand, utilizes electrical, thermal or acoustic energy to generate droplets in a continuous or on‐demand manner. DBB can be classified into inkjet bioprinting, electro‐hydrodynamic jetting, acoustic droplet ejection, and microvalve bioprinting. LBB utilizes laser power in one of two ways: photopolymerization, or cell transfer. Processes involving photopolymerization include SLA, DLP, and 2PP, and processes based on cell transfer include laser‐guidance direct writing and LIFT.
Figure 13. Classification of bioprinting technologies (adapted with permission from [81]).
Download figure:
Standard image High-resolution imageEBB has been used in the bioprinting of living cells since the early 2000s and continues to be utilized by the majority of the bioprinting community due to its simplicity, user friendliness, and the ability to generate scalable and structurally stable constructs in a relatively short time [82]. DBB, on the other hand, was first demonstrated by Klebe in 1988 with the use of an HP inkjet printer in order to pattern proteins, which subsequently evolved with the seminal cell printing work of Boland in 2003 [83]. Later, a number of studies have advanced DBB technology (such as the discovery of other DBB techniques), and the technology is currently preferred for medium/high resolution and high‐throughput applications, such as tissue models for drug screening and disease modelling. LBB was demonstrated for the first time by Odde and Renn in 1999 for the patterning of cells in primitive shapes, and it evolved further over the last 15 years with the adoption of SLA and LIFT techniques in bioprinting [84]. LBB is mainly used in applications requiring high‐precision patterning of cells, or high‐resolution fabrication of tissue constructs.
11.2. Current and future challenges
Although great progress has been made in bioprinting technologies over the last decade, each modality possesses its own limitations within various aspects, including process resolution, scalability, availability of compatible bioinks, and biomimicry. EBB technologies are compatible with a wide range of bioink materials (i.e. hydrogels and cell aggregates) and facilitate scalable constructs; however, these methods, in general, do not support process resolutions of higher than 100 μm. Thus, tissue models requiring smaller feature size, cellular heterogeneity, or gradients at a smaller length scale distance are not yet possible using EBB. DBB technologies, on the other hand, are highly suitable for high‐throughput applications, or applications requiring higher process resolution; however, they are impeded by two major challenges. First, only low-viscosity bioinks (such as hydrogel solutions in diluted form) are compatible with DBB as clogging in the nozzle and tubing system is a limitation. Cell density in the bioink is also limited to a few million cells per ml. Thus, the resultant tissue constructs are mechanically inferior and exhibit limited biomimicry as most tissues have higher cell densities (>107 cells ml−1) [83]. LBB technologies face several challenges, including biocompatibility of the photopolymerization process and related chemicals or cell transfer‐based processes. Both groups of processes are limited to a small set of compatible bioinks. Although photopolymerization processes yield constructs with well‐defined 3D shapes, these constructs often lack biomimicry and have a limited density of cells encapsulated. Processes based on cell transfer, in contrast, do not support fabrication of thick and structurally integrated constructs as compatible bioinks do not possess sufficient viscosity. The complexity of the laser and bioprinter setup limits their broader use. As the presented bioprinting approaches are complementary to each other, all aim towards the future goal of bioprinting, the achievement of clinically relevant, functional tissues and organs. One can expect seamless integration of these modalities into a single platform. Although there are a few commercial bioprinters providing such solutions at a primitive level, it can be expected that more integrated technologies will be available in the near future.
11.3. Advances in science and technology to meet challenges
Despite the several challenges experienced in bioprinting technologies, the field is growing, and a number of promising studies have recently been published that show some potential to overcome some of the aforementioned hurdles.
The Feinberg laboratory has introduced the FRESH method for EBB, which overcomes one of the major problems in building anatomically correct shapes of hydrogels. It can achieve high resolution compared to other biofabrication approaches [85]. In their study, they demonstrate the use of a gelatin support bath and utilized its Bingham plastic behaviour (figure 14(A)), where the bath acts like a solid at room temperature (supporting bioprinting) and liquid at 37 °C (supporting the removal of bioprinted constructs). In a similar purpose application, the Burdick laboratory developed in situ polymerization, where a wide range of photocurable hydrogels were crosslinked within the extrusion head and their rheological properties were tuned during bioprinting. This significantly improved the resolution and fidelity of bioprinted constructs (figure 14(B)) [17]. Such work demonstrated the effective coupling of EBB and photo‐crosslinking. The scalability problem of cell transfer‐based approaches (e.g. LIFT) has been mitigated by the work performed by Huang and co‐workers [86], where 3D volumetric tubular constructs, as shown in figure 14(C), were bioprinted by depositing alginate jets into a crosslinker vat with a levelling mechanism. It would be useful if further advancements were performed for other bioinks, particularly biologically useful bioinks (e.g. collagen) that crosslink slowly. On the other hand, the mechanical and structural integrity of tissue constructs fabricated via DBB has been ensured by integrating electrospinning [87], where bioink droplets were deposited onto electrospun fibres. A similar arrangement has been utilized in EBB of low viscous hydrogels or decellularized ECM components [88] with the integration of melt‐extruded polymeric frames. One of the contributing factors to the scalability problem is the limited viscosity of bioinks that current DBB technologies can tolerate. In this regard, the Lewis laboratory recently demonstrated acoustophoretic printing technology (see figure 14(D)). This new technology enables dispensing of droplets of high viscous inks (with viscosities up to 25 000 mPa·s) or yield stress fluids (τ0 > 50 Pa)) [89]. Further, the Lewis laboratory has demonstrated the application of acoustophoretic printing technology in bioprinting of an MSC‐laden 5 mg ml−1 collagen solution. Such an advancement has the potential to print droplets of other viscous bioinks, which is not quite feasible with existing DBB technologies.
Figure 14. (A) The FRESH bioprinting approach enabling plotting of bioink into a gelatin support bath, which acts like a Bingham plastic (solid at 22 °C and liquid at 37 °C) (adapted from [85]). (B) An in situ crosslink of photocrosslinkable gels facilitating bioprinting of high-fidelity constructs (adapted with permission from [17]). (C) The LIFT process with a support bath of crosslinking solution facilitates the bioprinting of branched vascular constructs (adapted with permission from [86]). (D) Acoustophoretic printing using sound waves to facilitate the deposition of droplets (adapted from [89]).
Download figure:
Standard image High-resolution image11.4. Concluding remarks
Although great progress has been made in bioprinting technologies over the last decade, each modality possesses its own limitations, including, but not limited to, process resolution, scalability, and availability of compatible bioinks; however, to date, the advances of each method seem to complement the shortcomings of other methods. Thus, to fill in the current technological gaps, it is likely that in the future technologies will encompass and utilize multiple modalities into single platforms along with the integration of novel processes, such as cell aggregate bioprinting techniques, in order to fabricate scalable, structurally‐stable, and perfusable tissue constructs with enhanced biomimicry and desired functionality.
Acknowledgments
This work has been supported by an NSF Award # 1624515, NIH Awards # R21 CA224422 01A1 and 1U19AI142733‐01, and a Hartz Family Professorship to I.T.O.